The Influenza Vaccine Puzzle: Challenges and Breakthrough
July 05, 2023
By Ryan M. Thomas
|
|
|
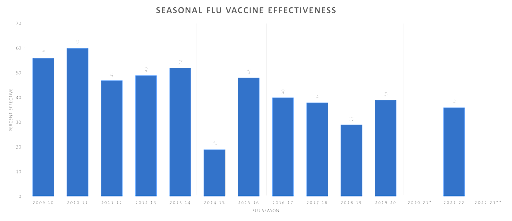
Influenza Vaccine Origins In 1938, a scientist named Jonas Salk, along with his team, successfully isolated the influenza virus and developed an inactivated vaccine. This initial vaccine was made from inactivated influenza virus grown in chicken eggs. Since then, some advancements have been made in influenza vaccine research and technology, but despite these efforts, the ongoing evolution and diversity of influenza viruses continue to present challenges in developing a universal and long-lasting vaccine. It’s been over 80 years since the first flu vaccine was developed yet, the disease still kills more than 400,000 people annually. In addition to the deaths, influenza is responsible for tens of billions of dollars in lost productivity and medical costs. While the flu might not feel like a top of mind medical or economic concern on a daily basis, how many of us would appreciate having those 4-5 sick days back per year? Or if we’re a business owner, to have an extra 5% of productivity from our workers? Influenza Vaccine Effectiveness Before the flu season, vaccine manufacturers attempt to identify the 2 to 4 most likely strains of influenza for the upcoming season. They then create a “high dose” multivalent vaccine with up to 4 flu strains. Because these legacy vaccine platforms are slow to develop and scale, predicting and selecting the most likely targets leads to very inconsistent results. According to the CDC, flu vaccine effectiveness against severe infections have failed to eclipse 50% over the last 7 years.
New Influenza Vaccine Platforms In 2021, the world’s largest influenza vaccine manufacturer agreed to a $3.2B acquisition of Translate Bio. Translate Bio developed an mRNA vaccine platform that promised to reduce the design and manufacturing time of influenza vaccines. Shortly after the acquisition, Sanofi abandoned their COVID mRNA clinical trial, the French Pharma company then quickly pivoted towards influenza. Being the world’s largest flu vaccine manufacturer, the company decided to test their new mRNA platform against their legacy platform which creates more than $3B in revenue for the company on an annual basis. Last week, Sanofi admitted similar disappointing results to what we’ve seen with other first generation mRNA platforms. In Sanofi’s investor presentation, they concluded that first-generation mRNA vaccines “will not win” against the flu. If we’re intellectually honest, we can also come to the same conclusion with COVID-19 mRNA vaccines. At best, they’ve bought us a stalemate. Influenza Vaccine Challenges If we’ve had 80 years to develop more effective vaccines, why has influenza posed such a challenge? We eradicated smallpox, why can’t we do the same for the flu? Scientists typically identify 3 main areas that pose a challenge to the development of an effective flu vaccine:
The nature of influenza viruses and their ability to undergo rapid genetic changes through antigenic (An antigen is a substance that is capable of stimulating an immune response in the body. It can be any foreign substance, such as a protein, carbohydrate, or even a small molecule, that is recognized by the immune system as “non-self” and triggers an immune response. Antigens can come from various sources, including pathogens like viruses and bacteria, as well as non-infectious substances such as pollen, dust, or certain chemicals) drift and shift. These antigen changes negatively affect a vaccine’s ability to mount a protective response against the disease.
Best selling influenza vaccines still use a complex and time-consuming process including the propagation of the virus in eggs. The limitations associated with egg-based production and the ability to quickly respond to strain adaptation limited speed and scalability.
The challenge of accurately predicting the predominant influenza strain each season requires a combination of epidemiological data, surveillance, and strain characterization. A failure in any of these areas can lead to a vaccine with very low effectiveness that season and result in the unnecessary deaths of thousands. Now, these are just the 3 challenging areas to develop an effective flu vaccine – we haven’t even discussed the challenges of developing a universal vaccine. The Promise of mRNA Technology Sanofi, Moderna, Pfizer and others have jumped head first into the rapid development of mRNA influenza vaccines. The first-generation technology solves the issue of more rapid manufacturing by bypassing the need for the propagation of the virus in eggs. This advancement means that the time from strain identification to manufacturing is greatly reduced, decreasing the potential error for identifying the wrong dominant strain for the season. As first-generation mRNA vaccines have rolled out in the clinic, it’s becoming clear that mRNA platforms hold promise, but have not yet advanced to overcome the issue of identifying multiple antigens to create long-term protection and potential immunity against the disease. Eyam’s Next Generation Influenza Technologies At Eyam, Dr. Jefferies and team have anticipated many of these challenges. With the development of the Jennerator Platform, we have an AI-driven design platform that crunches data on millions of sequences of the virus to identify those critical antigen targets and incorporate immune system markers (these help create immune responses for people that typically don’t respond well to traditional vaccines). Design is only one competent of an effective vaccine. After the Jennerator designs the vaccine payloads, the Gemini Platform delivers them. Gemini was created to overcome the limitations of delivering a small payload (think only a couple of antigens). With its robust payload expression of more than 42 days (7-10x longer than first generation mRNA platforms), the immune system has a greater opportunity to mount a stronger response to create protective antibodies as well as t and b cells. We’re also working with our manufacturing partners to reduce the time of production using novel methods that have the potential to significantly reduce the time from design, testing and production to quickly roll out new, longer lasting and protective vaccines. |
The Influenza Vaccine Puzzle: Challenges and Breakthrough
July 05, 2023
By Ryan M. Thomas
|
|
|
Influenza Vaccine Origins In 1938, a scientist named Jonas Salk, along with his team, successfully isolated the influenza virus and developed an inactivated vaccine. This initial vaccine was made from inactivated influenza virus grown in chicken eggs. Since then, some advancements have been made in influenza vaccine research and technology, but despite these efforts, the ongoing evolution and diversity of influenza viruses continue to present challenges in developing a universal and long-lasting vaccine. It’s been over 80 years since the first flu vaccine was developed yet, the disease still kills more than 400,000 people annually. In addition to the deaths, influenza is responsible for tens of billions of dollars in lost productivity and medical costs. While the flu might not feel like a top of mind medical or economic concern on a daily basis, how many of us would appreciate having those 4-5 sick days back per year? Or if we’re a business owner, to have an extra 5% of productivity from our workers? Influenza Vaccine Effectiveness Before the flu season, vaccine manufacturers attempt to identify the 2 to 4 most likely strains of influenza for the upcoming season. They then create a “high dose” multivalent vaccine with up to 4 flu strains. Because these legacy vaccine platforms are slow to develop and scale, predicting and selecting the most likely targets leads to very inconsistent results. According to the CDC, flu vaccine effectiveness against severe infections have failed to eclipse 50% over the last 7 years.
New Influenza Vaccine Platforms In 2021, the world’s largest influenza vaccine manufacturer agreed to a $3.2B acquisition of Translate Bio. Translate Bio developed an mRNA vaccine platform that promised to reduce the design and manufacturing time of influenza vaccines. Shortly after the acquisition, Sanofi abandoned their COVID mRNA clinical trial, the French Pharma company then quickly pivoted towards influenza. Being the world’s largest flu vaccine manufacturer, the company decided to test their new mRNA platform against their legacy platform which creates more than $3B in revenue for the company on an annual basis. Last week, Sanofi admitted similar disappointing results to what we’ve seen with other first generation mRNA platforms. In Sanofi’s investor presentation, they concluded that first-generation mRNA vaccines “will not win” against the flu. If we’re intellectually honest, we can also come to the same conclusion with COVID-19 mRNA vaccines. At best, they’ve bought us a stalemate. Influenza Vaccine Challenges If we’ve had 80 years to develop more effective vaccines, why has influenza posed such a challenge? We eradicated smallpox, why can’t we do the same for the flu? Scientists typically identify 3 main areas that pose a challenge to the development of an effective flu vaccine:
The nature of influenza viruses and their ability to undergo rapid genetic changes through antigenic (An antigen is a substance that is capable of stimulating an immune response in the body. It can be any foreign substance, such as a protein, carbohydrate, or even a small molecule, that is recognized by the immune system as “non-self” and triggers an immune response. Antigens can come from various sources, including pathogens like viruses and bacteria, as well as non-infectious substances such as pollen, dust, or certain chemicals) drift and shift. These antigen changes negatively affect a vaccine’s ability to mount a protective response against the disease.
Best selling influenza vaccines still use a complex and time-consuming process including the propagation of the virus in eggs. The limitations associated with egg-based production and the ability to quickly respond to strain adaptation limited speed and scalability.
The challenge of accurately predicting the predominant influenza strain each season requires a combination of epidemiological data, surveillance, and strain characterization. A failure in any of these areas can lead to a vaccine with very low effectiveness that season and result in the unnecessary deaths of thousands. Now, these are just the 3 challenging areas to develop an effective flu vaccine – we haven’t even discussed the challenges of developing a universal vaccine. The Promise of mRNA Technology Sanofi, Moderna, Pfizer and others have jumped head first into the rapid development of mRNA influenza vaccines. The first-generation technology solves the issue of more rapid manufacturing by bypassing the need for the propagation of the virus in eggs. This advancement means that the time from strain identification to manufacturing is greatly reduced, decreasing the potential error for identifying the wrong dominant strain for the season. As first-generation mRNA vaccines have rolled out in the clinic, it’s becoming clear that mRNA platforms hold promise, but have not yet advanced to overcome the issue of identifying multiple antigens to create long-term protection and potential immunity against the disease. Eyam’s Next Generation Influenza Technologies At Eyam, Dr. Jefferies and team have anticipated many of these challenges. With the development of the Jennerator Platform, we have an AI-driven design platform that crunches data on millions of sequences of the virus to identify those critical antigen targets and incorporate immune system markers (these help create immune responses for people that typically don’t respond well to traditional vaccines). Design is only one competent of an effective vaccine. After the Jennerator designs the vaccine payloads, the Gemini Platform delivers them. Gemini was created to overcome the limitations of delivering a small payload (think only a couple of antigens). With its robust payload expression of more than 42 days (7-10x longer than first generation mRNA platforms), the immune system has a greater opportunity to mount a stronger response to create protective antibodies as well as t and b cells. We’re also working with our manufacturing partners to reduce the time of production using novel methods that have the potential to significantly reduce the time from design, testing and production to quickly roll out new, longer lasting and protective vaccines. |