Vaccine Platforms: Looking Beyond the Pandemic and COVID-19
September 30, 2022
By Ryan M. Thomas
This past month, President Joe Biden declared the end of the COVID-19 Pandemic. After his comments sparked backlash from infectious disease experts that pointed out that more than 400 people per day are still dying of COVID-19 in the US alone, and that the disease is trending to kill an average of 150,000 people per year in the US (equivalent to 3 bad flu years), he corrected himself saying that we are in a better position than at any time since the onset of the pandemic. While new data is emerging about the long-term neurological effects of COVID-19, even Biden’s corrected statement is debatable.
Nevertheless, it’s worth examining Eyam’s future in a post-pandemic world.
A Platform Company
First, it’s important to understand the difference between a “COVID-19 Company” and a “Platform Biotech” working on COVID-19.
At Eyam, we’ve developed a proprietary vaccine platform with novel viral vectors that improve vaccine delivery and protein expression. Combined with our self-amplifying technologies, that means that we can quickly design and code a payload to protect and defend against novel, emerging diseases so that our immune systems can be protected long-term against new threats.
Over the last two years, we’ve optimized our vaccine platform, validated its effectiveness and tested dozens of COVID-19 vaccine candidates. We’ve also recently acquired an option to license a cancer antibody biologic which has shown promising data in eliminating certain cancers in animals. Utilizing our Jennerator bioinformatics platform, we’ve also designed new vaccines for influenza which we aim to begin testing before the end of 2022.
To understand this better, let’s go through a little Vaccine 101.
Vaccine 101
Essentially, a vaccine “is a suspension of weakened, killed, or fragmented microorganisms or toxins or other biological preparation, such as those consisting of antibodies, lymphocytes, or mRNA, that is administered primarily to prevent disease” (Britannica). The goal of a vaccine is to create active immunity such that when we come into contact with a virus, our immune system will have antibodies and other defenses to trigger the prevention of an infection. Natural immunity occurs when we have been previously infected and our bodies create an immune response to prevent future infection or reduce the severity of a future infection. A virus’ ability to mutate will affect both a vaccine’s and natural immunity’s ability to protect against future infection.
In the case of the common flu, the virus has an ability to evade our immune system’s defenses on a regular basis, permitting the virus to penetrate our defenses. A seasonal flu shot, does prime the body and create new antibodies that reduce the likelihood of infection and severity of symptoms.
Vaccine History
Dr. Edward Jenner is credited for his groundbreaking work on vaccines and his contribution to eradicating smallpox. While he wasn’t the first to begin work on vaccines, or even to suggest that exposing people to cowpox (a less deadly vision also in the smallpox family) he did make important contributions that lead to the mass adoption of vaccines.
Jenner made the connection early on that exposure to less deadly viruses from the same family of viruses could create immune system defenses that would reduce the severity of infection.
These early vaccines were some of the greatest contributors to increased life expectancy and quality of life.
The process of making traditional vaccines is long and complicated. For years, there has been hope that the creation of an mRNA vaccine platform would reduce the turnaround time and permit scientists to create better vaccines that could protect against viruses that had evaded our ability to create vaccines.
Traditional vs. mRNA Vaccines
Traditional vs. mRNA vaccines – what makes mRNA vaccines different from traditional vaccines?
- Traditional vaccine: Traditional vaccines work by helping your body develop antibodies, allowing you to fight off infection. There are four main types: Live-attenuated vaccines (chickenpox, measles, mumps, rubella), inactivated vaccines (Hepatitis A, flu shot, rabies), subunit, recombinant, polysaccharide, and conjugate vaccines (shingles, HPV, whooping cough), toxoid vaccines (tetanus, diphtheria)
- mRNA vaccines: They work differently than traditional vaccinations. Rather than introducing a virus into your system, messenger ribonucleic acid (mRNA) works with the cells that make proteins in your body. The mRNA teaches those cells how to make a specific protein to create an immune response against a particular sickness, such as COVID-19.
- Eyam’s self amplifying mRNA platform: Self amplifying technology reduces the amount of RNA in the vaccine and expresses it over much longer time for greater safety and longer lasting protection.
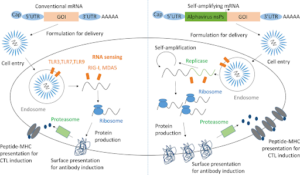
Vaccine Platforms
Eyam’s vaccine platform allows for a “plug and play” physical framework that can be used when developing vaccines for emerging infectious diseases. Using a base carrier, or “vehicle,” such as a nucleic acid, viral vector, or liposome, permits the platform to work interchangeably for various diseases. Extending the definition to pharma, a vaccine platform is any underlying technology — a mechanism, delivery method, or cell line — that can be used to develop multiple vaccines.
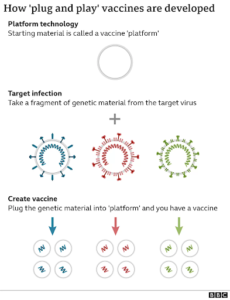
While we’ve previously shared a lot about our COVID-19 vaccines, we’ve actually been hard at work optimizing a new vaccine platform that will allow us to streamline design and development of vaccines for various indications (viruses) in the future.
The old-school method of developing vaccines means you have to go back to the raw materials and start from scratch for every vaccine you make. It is like starting with a bench of flour, sugar, eggs and butter. The next step is to take the offending virus, or other disease-causing microbes, and either kill it or weaken it to make a vaccine.
Eyam’s “Plug-and-play” platform’s advantage is that most of the work has already been done – the cake has been pre-baked, it just needs to be “decorated” in order to match its target.
This flexibility will allow us to cost effectively and quickly move to develop new vaccines in the future.
Once designed and licensed for one vaccine, the development of future vaccines using the same platform would simply require substituting the desired antigenic component, or a genetic compound that normally triggers an immune response. This could enable faster and lower cost development, regulatory approval and mass production.
Next Steps
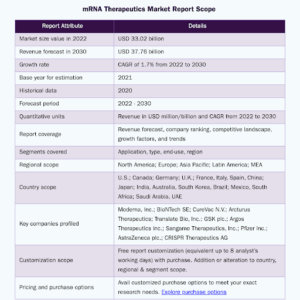
The mRNA vaccine platform has become a hammer that pharma companies are racing to throw new nails under. One of these nails is influenza, which the World Health Organization estimates infects 1 billion people worldwide every year despite existing vaccines.
- The influenza vaccine market is expected to grow from US$ 7.5B in 2021 to US$ 12.2B by 2028.
- Increasing government support to promote influenza vaccination and rising investment by top market players and governments are the major factors boosting the market.
- COVID-19 products account for the vast majority of prophylactic vaccine revenues in the near term, while other vaccines for illnesses including influenza and respiratory syncytial virus also contribute to the segment growth.
Additionally, a rise in the number of patients with cancer, influenza, infectious diseases, and respiratory illnesses is expected to fuel the market growth. For instance, according to an NCBI report, in 2019, the WHO estimates that up to 500,000 people worldwide die from influenza each year and approximately one billion people are sick, thus contributing to increases in health care costs and productivity loses to companies and lower economic output for entire countries.
Just as Dr. Jenner helped bring vaccines to mainstream adoption, mRNA vaccines have not become accepted for their powerful new applications. Eyam’s vaccine platform paired with our Jennerator bioinformatics platform hold exciting promise to both design next generation vaccines and “plug and play” them into our platform.
The future is bright and the opportunities are many.
Vaccine Platforms: Looking Beyond the Pandemic and COVID-19
September 30, 2022
By Ryan M. Thomas
This past month, President Joe Biden declared the end of the COVID-19 Pandemic. After his comments sparked backlash from infectious disease experts that pointed out that more than 400 people per day are still dying of COVID-19 in the US alone, and that the disease is trending to kill an average of 150,000 people per year in the US (equivalent to 3 bad flu years), he corrected himself saying that we are in a better position than at any time since the onset of the pandemic. While new data is emerging about the long-term neurological effects of COVID-19, even Biden’s corrected statement is debatable.
Nevertheless, it’s worth examining Eyam’s future in a post-pandemic world.
A Platform Company
First, it’s important to understand the difference between a “COVID-19 Company” and a “Platform Biotech” working on COVID-19.
At Eyam, we’ve developed a proprietary vaccine platform with novel viral vectors that improve vaccine delivery and protein expression. Combined with our self-amplifying technologies, that means that we can quickly design and code a payload to protect and defend against novel, emerging diseases so that our immune systems can be protected long-term against new threats.
Over the last two years, we’ve optimized our vaccine platform, validated its effectiveness and tested dozens of COVID-19 vaccine candidates. We’ve also recently acquired an option to license a cancer antibody biologic which has shown promising data in eliminating certain cancers in animals. Utilizing our Jennerator bioinformatics platform, we’ve also designed new vaccines for influenza which we aim to begin testing before the end of 2022.
To understand this better, let’s go through a little Vaccine 101.
Vaccine 101
Essentially, a vaccine “is a suspension of weakened, killed, or fragmented microorganisms or toxins or other biological preparation, such as those consisting of antibodies, lymphocytes, or mRNA, that is administered primarily to prevent disease” (Britannica). The goal of a vaccine is to create active immunity such that when we come into contact with a virus, our immune system will have antibodies and other defenses to trigger the prevention of an infection. Natural immunity occurs when we have been previously infected and our bodies create an immune response to prevent future infection or reduce the severity of a future infection. A virus’ ability to mutate will affect both a vaccine’s and natural immunity’s ability to protect against future infection.
In the case of the common flu, the virus has an ability to evade our immune system’s defenses on a regular basis, permitting the virus to penetrate our defenses. A seasonal flu shot, does prime the body and create new antibodies that reduce the likelihood of infection and severity of symptoms.
Vaccine History
Dr. Edward Jenner is credited for his groundbreaking work on vaccines and his contribution to eradicating smallpox. While he wasn’t the first to begin work on vaccines, or even to suggest that exposing people to cowpox (a less deadly vision also in the smallpox family) he did make important contributions that lead to the mass adoption of vaccines.
Jenner made the connection early on that exposure to less deadly viruses from the same family of viruses could create immune system defenses that would reduce the severity of infection.
These early vaccines were some of the greatest contributors to increased life expectancy and quality of life.
The process of making traditional vaccines is long and complicated. For years, there has been hope that the creation of an mRNA vaccine platform would reduce the turnaround time and permit scientists to create better vaccines that could protect against viruses that had evaded our ability to create vaccines.
Traditional vs. mRNA Vaccines
Traditional vs. mRNA vaccines – what makes mRNA vaccines different from traditional vaccines?
- Traditional vaccine: Traditional vaccines work by helping your body develop antibodies, allowing you to fight off infection. There are four main types: Live-attenuated vaccines (chickenpox, measles, mumps, rubella), inactivated vaccines (Hepatitis A, flu shot, rabies), subunit, recombinant, polysaccharide, and conjugate vaccines (shingles, HPV, whooping cough), toxoid vaccines (tetanus, diphtheria)
- mRNA vaccines: They work differently than traditional vaccinations. Rather than introducing a virus into your system, messenger ribonucleic acid (mRNA) works with the cells that make proteins in your body. The mRNA teaches those cells how to make a specific protein to create an immune response against a particular sickness, such as COVID-19.
- Eyam’s self amplifying mRNA platform: Self amplifying technology reduces the amount of RNA in the vaccine and expresses it over much longer time for greater safety and longer lasting protection.

Vaccine Platforms
Eyam’s vaccine platform allows for a “plug and play” physical framework that can be used when developing vaccines for emerging infectious diseases. Using a base carrier, or “vehicle,” such as a nucleic acid, viral vector, or liposome, permits the platform to work interchangeably for various diseases. Extending the definition to pharma, a vaccine platform is any underlying technology — a mechanism, delivery method, or cell line — that can be used to develop multiple vaccines.

While we’ve previously shared a lot about our COVID-19 vaccines, we’ve actually been hard at work optimizing a new vaccine platform that will allow us to streamline design and development of vaccines for various indications (viruses) in the future.
The old-school method of developing vaccines means you have to go back to the raw materials and start from scratch for every vaccine you make. It is like starting with a bench of flour, sugar, eggs and butter. The next step is to take the offending virus, or other disease-causing microbes, and either kill it or weaken it to make a vaccine.
Eyam’s “Plug-and-play” platform’s advantage is that most of the work has already been done – the cake has been pre-baked, it just needs to be “decorated” in order to match its target.
This flexibility will allow us to cost effectively and quickly move to develop new vaccines in the future.
Once designed and licensed for one vaccine, the development of future vaccines using the same platform would simply require substituting the desired antigenic component, or a genetic compound that normally triggers an immune response. This could enable faster and lower cost development, regulatory approval and mass production.
Next Steps
The mRNA vaccine platform has become a hammer that pharma companies are racing to throw new nails under. One of these nails is influenza, which the World Health Organization estimates infects 1 billion people worldwide every year despite existing vaccines.
- The influenza vaccine market is expected to grow from US$ 7.5B in 2021 to US$ 12.2B by 2028.
- Increasing government support to promote influenza vaccination and rising investment by top market players and governments are the major factors boosting the market.
- COVID-19 products account for the vast majority of prophylactic vaccine revenues in the near term, while other vaccines for illnesses including influenza and respiratory syncytial virus also contribute to the segment growth.
Additionally, a rise in the number of patients with cancer, influenza, infectious diseases, and respiratory illnesses is expected to fuel the market growth. For instance, according to an NCBI report, in 2019, the WHO estimates that up to 500,000 people worldwide die from influenza each year and approximately one billion people are sick, thus contributing to increases in health care costs and productivity loses to companies and lower economic output for entire countries.

Just as Dr. Jenner helped bring vaccines to mainstream adoption, mRNA vaccines have not become accepted for their powerful new applications. Eyam’s vaccine platform paired with our Jennerator bioinformatics platform hold exciting promise to both design next generation vaccines and “plug and play” them into our platform.
The future is bright and the opportunities are many.



